- Consumer Goods
- Performance Materials
- Technology
-
Services
- Overview
- Sourcing
Sourcing
Accessing a global sourcing network.
- Market insights
Market insights
Generating ideas for growth.
- After-sales services
After-sales services
Servicing throughout the entire lifespan of your product.
- Regulatory affairs
Regulatory
Overcoming regulatory challenges.
- 日本企業海外進出支援
日本企業海外進出支援
ジャパンアウトバウンドサービス
- Insights
- Home


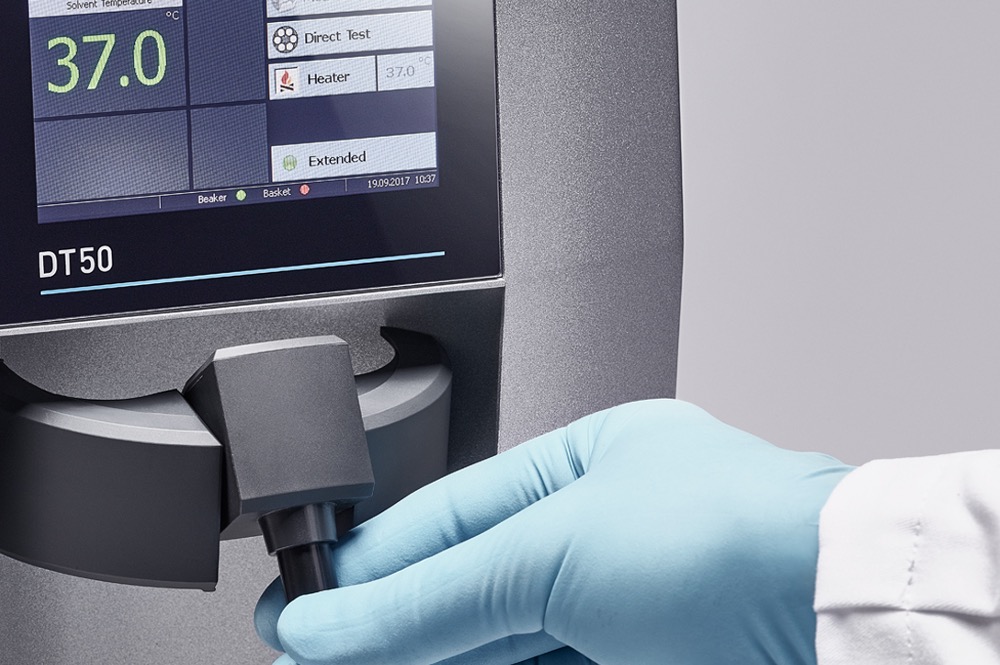






 SOTAX leads the way in the development and manufacture of high-quality pharmaceutical testing equipment and associated services. Our equipment is used worldwide in the development and production processes of our customers and the pharmaceutical and science industries. SOTAX develops, produces and markets system solutions for pharmaceutical testing and does so in close cooperation with pharmaceutical and scientific partners.
SOTAX leads the way in the development and manufacture of high-quality pharmaceutical testing equipment and associated services. Our equipment is used worldwide in the development and production processes of our customers and the pharmaceutical and science industries. SOTAX develops, produces and markets system solutions for pharmaceutical testing and does so in close cooperation with pharmaceutical and scientific partners.


