- ผลิตภัณฑ์อุปโภคบริโภค
- ผลิตภัณฑ์เพื่อสุขภาพ
- วัตถุดิบอุตสาหกรรม
- เทคโนโลยี
-
บริการของเรา
- ภาพรวม
- การจัดหาแหล่งผลิต
การจัดหาแหล่งผลิต
เพิ่มโอกาสเพื่อเข้าถึงเครือข่ายแหล่งผลิตจากทั่วโลก
- การวิจัยและวิเคราะห์
การวิจัยและวิเคราะห์
เราเพิ่มประสิทธิภาพเพื่อการเติบโตของธุรกิจของคุณ
- การตลาดและการขาย
การตลาดและการขาย
เราเพิ่มโอกาสทางรายได้ให้ธุรกิจของคุณ
- การกระจายสินค้าและโลจิสติกส์
การกระจายสินค้าและโลจิสติกส์
เราจัดส่งสินค้าได้ทุกที่และทุกเวลาที่คุณต้องการ
- การบริการหลังการขาย
การบริการหลังการขาย
เราให้บริการคุณในตลอดช่วงการใช้ผลิตภัณฑ์
- ข้อมูลเชิงลึก
-
Thailand
-
EN | ไทย
-
Search
- กลุ่มดีเคเอสเอช
- ติดต่อ
- ร่วมงานกับเรา
- นักลงทุน
- หน้าแรก
- เทคโนโลยี
- ผลิตภัณฑ์ของเรา
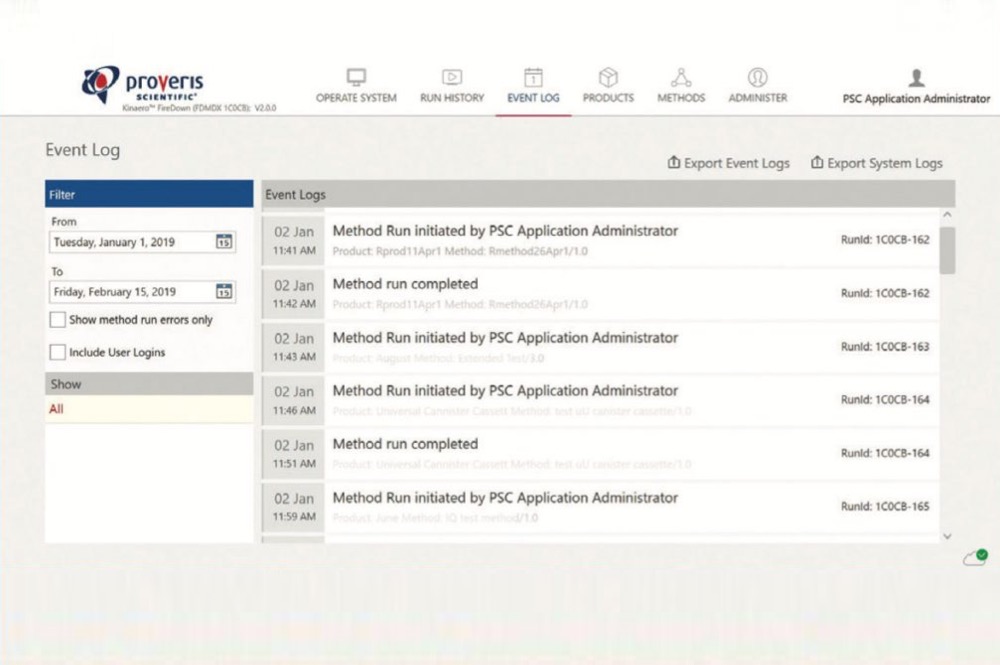
- Proveris Scientific - Kinaero™ High-Throughput pMDI Fire-Down System
- หน้าแรก
- เทคโนโลยี
- ผลิตภัณฑ์ของเรา
- Proveris Scientific - Kinaero™ High-Throughput pMDI Fire-Down System