However, the technique only works well for fully oxidized samples, and can be tricky for semi-oxidized or reduced/elemental samples without a proper recipe. Damage to the platinumwares and instrument can lead to costly replacement parts, and care should be taken to minimize these risks.

Figure 1: LeNeo - The cost effective automatic electric fusion machine
The fusion of sulfide ores and minerals are risky to be processed in a platinum crucible generally, due to the content of reduced form of Iron elements, as well as the presence of high amounts of Sulfur. The platinum crucible could be tarnished, or potentially ruined even in a single fusion cycle (see Figure 2).

Figure 2: Damaged crucible due to the fusion of sulfidic ores
The following issues was brought up to be looked into:
- Direct fusion of iron ore for certain ores causes damage to the crucible (magnetite & High Sulfur)
- Pre-treatment for these ores take a long time (total preparation time approximately 3h, from start to end)
A short case study was proposed with the following objectives:
- To shorten the pre-treatment time where possible
- To employ direct fusion where possible.
- Avoid any damage to the platinumware at the end of the fusion cycle
Analytical requirements in terms of retention of Sulfur was not explored in this study. Further optimization to the oxidation process and oxidizer type can be explored to look into the retention of sulfur.
In this study, a single position fully automatic fusion instrument capable of up to 1200°C is used. The LeNeo is a fully automatic cold to cold fusion instrument, with virtually unlimited programmable heating and cooling steps, allowing for a highly reproducible and reliable sample preparation step. A set of samples from a mining site consisting of low grade iron ore, magnetite, and iron sulfide was provided for this study. A standalone muffle furnace was also used to study alternative pre-treatment steps.
For the chemicals, pre-fused version of Lithium Tetraborate/Lithium Metaborate/Lithium Iodide in the ratio of 49.75/49.75/0.5 was used. Ammonium Nitrate was employed as the oxider in this case study.
In this case study, ammonium nitrate was chosen as the reaction oxidizer. The following reaction equation of ammonium nitrate and pyrite was proposed by Nakamura et.al., 1994:
2 FeS2 + 11 NH4NO3 → 0.55 Fe2O3 + 0.88 (NH4)Fe(SO4)2 + 2.02 SO2 + 19.25 H2O
+0.02 FeS2 + 0.1 NH4NO3 + 0.18 (NH4)2SO4 + 10.28 N2 + 0.43 H2 - - - (1)
The reaction of magnetite (Fe2+(Fe3+)2.(O2-)4) is assumed to be simpler, with the oxidation of Fe2+ to Fe3+, with oxygen provided for from the environment, as well as the decomposition of ammonium nitrate. The reaction products are not verified in this study.
4 Fe3O4 + O2 → 6 Fe2O3 - - - (2)
The pre-fused fluxes are typically of the size between 0.3 to 0.5mm in diameter, as compared to about 30μm for powdered fluxes (Willis, 2010).
4 LiI + 2 NH4NO3 → 2 Li2O + 2 I2 + 2 N2 + 4 H2O - - - (3)
The oxidation of iodide salts is a negative side effect of the addition of oxidizers, which will impact the pouring and removal of fused beads. The incorporation of iodide salts as a pre-fused mixture within the flux beads greatly reduces this side effects, shielding the iodide salts from the oxidative properties of Nitrates at lower temperatures, prior to the melting point of the Lithium Borate fluxes.
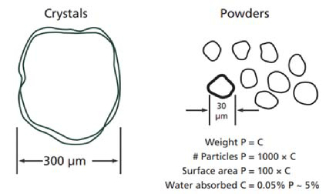
Figure 3: Prefused bead fluxes (left) vs. Powdered fluxes (right). Figure taken from (Willis, 2010)
The sample pre-treatment time was successfully reduced from about 2.5 hours to just 10 minutes in the presence of ammonium nitrate. For the magnetite samples, 1.5g of samples and 1.65g of ammonium nitrate was mixed together in a ceramic crucible and heated in a muffle furnace at 500°C for 10 minutes. For sulfidic ores, 1.5g of sample was mixed with 3.5g of ammonium nitrate, and the mixture was heated in a muffle furnace at 500°C for 15 minutes.
Changes in masses are noted down to calculate and correct for gain/loss in masses during the reaction process.
The products can then be fused in the usual general oxides program (sample: flux ratio of 1:10), with a slight excess of ammonium nitrate in proportion to the sample’s weight, with the overall general oxides program time approximately 24 minutes. This results in a total time sample preparation time of slightly above 0.5 hours for both pre-treatment and fusion. The results of this process are provided in Figure 5 & Figure 4. In both cases, there is no evident of tarnish on the crucible after fusion (before washing with acid), and the fusion mixture pours completely from the crucible into the mold. The fused bead was also easily removed from the mold.

Figure 5: Fusion of Iron Sulfide ore (left) and Figure 4: Fusion of Magnetite ore (right)
This short case study shows the feasibility of shortening the process with the correct recipe. Further development can be carried out for fully direct fusion, without the use of a muffle furnace, through the use of special crucible set-up. Additionally, further studies on the retention of Sulfur can be carried out, should the analytical requirement arise.
References:
- Nakamura, H., Iwasaki, M., Sato, S., & Hara, Y. (1994). The reaction of ammonium nitrate with pyrite. Journal of Hazardous Materials, 293-303.
- Willis, J. P. (2010). XRF SAMPLE PREPARATION, Glass beads by Borate Fusion. Almelo, The Netherlands: PANalytical B.V.